Unravelling the impact of electrolyte nature on Sn4P3/C negative electrodes for Na-ion batteries†
Abstract
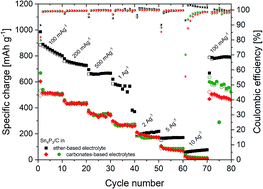
Carbon coated tin phosphide is synthesized by an easily scalable ball milling method. The electrochemical performance of Sn4P3/C electrodes is explored using different combinations of carbonate and ether-based solvents. Our experiments reveal that a remarkable improvement in the specific charge of Sn4P3 anodes can be obtained by using a combination of NaPF6 salt and diethylene glycol dimethyl ether electrolyte. A reversible specific charge of 900 mA h g−1 and initial coulombic efficiency of around 90% have been achieved. We carefully examine the origin of the improved capacity and cycle life when using glyme-based electrolytes and compare with carbonate electrolytes. Moreover, the reaction mechanism of the Sn based anode is further discussed.


 Please wait while we load your content...
Please wait while we load your content...