Polyethylenimine-modified nickel phosphide nanosheets: interfacial protons boost the hydrogen evolution reaction†
Abstract
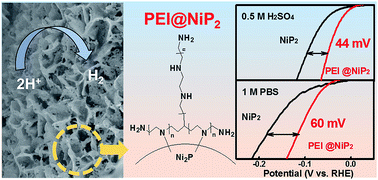
The hydrogen evolution reaction (HER) is an important half-reaction in water electrolysis. According to the Nernst equation, increasing the interfacial proton concentration may be an effective strategy for reducing the overpotential of HER. Herein, based on the interface engineering strategy, we successfully synthesized polyethyleneimine (PEI)-modified nickel phosphide (NiP2) nanosheets on carbon cloth (CC) nanohybrids (denoted as PEI@NiP2-CC). Multiple physical characterizations proved that the PEI molecules are firmly anchored on the NiP2 surface due to strong N–Ni interactions. In both acidic and neutral media, the solution protons increased at the PEI@NiP2-CC/electrolyte interface due to the protonation of the –NH2 groups in the PEI molecules. The effect of the molecular weight (MW) of PEI on the HER activity was explored in detail. The MW-optimized PEI@NiP2-CC nanohybrids showed only 44 and 100 mV overpotentials for HER in acidic and neutral media, respectively, and were much better than un-modified NiP2-CC nanohybrids. This work provides a strong basis for determining the correlativity between surface modification and catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...