Electron distribution tuning of fluorine-doped carbon for ammonia electrosynthesis†
Abstract
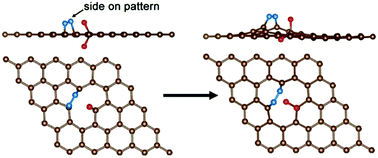
The electrochemical N2 reduction reaction (N2RR), as a key reaction to realize the electrosynthesis of ammonia under ambient conditions, is significantly inhibited by the low efficiency of electrocatalysts. In this study, we report the improvement of the N2RR efficiency using a nonmetal, fluorine-doped carbon. The fluorine doping enables the tuning of the electron distribution of carbon, which provides positively charged carbon sites that are prone to adsorb N2 than H+ under acidic aqueous conditions. Density-functional theory calculations indicate that the N2 molecule is adsorbed on the two contrapuntal carbon atoms in the form of a side-on pattern, and the pathway of N2 turning to NH3 has the lowest energy barrier. The fluorine-doped carbon exhibits better N2RR performance than the undoped carbon, with a peak ammonia production rate (6.9 μg h−1 cm−2) and a corresponding high faradaic efficiency (12.1%) at −0.55 V versus the reversible hydrogen electrode (RHE). Our work shows the attractive features of developing doped carbon materials as nonmetal N2RR electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...