Pyrite-type ruthenium disulfide with tunable disorder and defects enables ultra-efficient overall water splitting†
Abstract
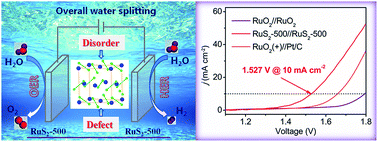
The exploration of efficient electrocatalysts for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is significant for water splitting associated with the storage of clean and renewable energy. Here, we report a simple and scalable low-temperature sulfuration method to achieve simultaneous modulation of disorder and defects in pyrite-type RuS2 nanoparticles to dramatically enhance the HER and OER catalytic activity. The disordered structure can increase the electrochemically active surface area, while defect engineering can effectively regulate the electronic structure and thus improve the intrinsic activity, as revealed by combined experimental and theoretical density functional theory (DFT) investigations. Through controllable disorder and defect engineering, the optimized RuS2-500 catalyst (with a sulfuration temperature of 500 °C) supported on a glassy carbon electrode exhibits ultra-efficient bifunctional electrocatalytic activity with η−10 = 78 mV for the HER and η10 = 282 mV for the OER, superior to various Ru-based and pyrite-type catalysts. Remarkably, when used as both the anode and the cathode in an alkaline water electrolyzer, RuS2-500 delivers 10 mA cm−2 at an ultralow cell voltage of 1.527 V with long-term stability, outperforming the benchmark Pt/C//RuO2 couple and most state-of-the-art overall-water-splitting electrocatalysts ever reported. This work thus provides a new and facile way for improving the catalytic activity through a synergistic modulation strategy.


 Please wait while we load your content...
Please wait while we load your content...