Mapping the sodium intercalation mechanism, electrochemical properties and structural evolution in non-stoichiometric alluaudite Na2+2δFe2−δ(SO4)3 cathode materials†
Abstract
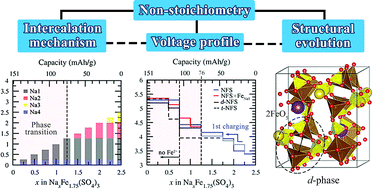
In the scientific advancement of future cathode materials, alluaudite sodium iron sulfate Na2+2δFe2−δ(SO4)3 (NxFyS) has emerged as one of the most promising candidates for sustainable sodium-ion batteries due to its high Fe2+/3+ redox potential (3.8 V vs. Na/Na+), low cost, and high rate capability. Usually, this material occurs in a non-stoichiometric form with partial Na+ substitutions on Fe sites, where δ is close to 0.25 (N2.5F1.75S) depending on the synthesis conditions. While many contemporary works have primarily been directed to study this non-stoichiometric compound, our previous theoretical prediction unveiled the possibility to synthesize stoichiometric alluaudite (N2F2S), which is expected to deliver higher specific capacity (∼120 mA h g−1) as compared to the non-stoichiometric derivatives. This provokes curiosity toward the non-stoichiometric effect on the electrochemical activities and sodium intercalation mechanism in alluaudite materials. In this work, we therefore perform rigorous first-principles calculations to study the structural evolution, electrochemical behavior, and voltage profile of NxFyS with y = 2, 1.75, and 1.5. We reveal the likelihood of two phase transitions after half desodiation process, whereas the probability is reduced with a higher degree of non-stoichiometry, suggesting improvement in the structural reversibility for N2.5F1.75S and N3F1.5S. The prediction of the voltage profiles shows the benefit of non-stoichiometry in enhancing the specific capacity and identifies the structural rearrangement of Fe2O10 dimers as the hidden reason behind the irreversible sharp peak experimentally observed in differential galvanostatic profiles.


 Please wait while we load your content...
Please wait while we load your content...