SnSb vs. Sn: improving the performance of Sn-based anodes for K-ion batteries by synergetic alloying with Sb†
Abstract
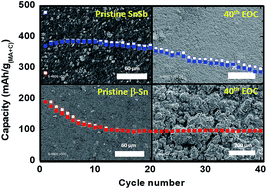
The electrochemical mechanism and performance of Sn-based electrodes are thoroughly studied in K-ion batteries. Low temperature ex situ119Sn Mössbauer spectroscopy combined with first principles calculations provides a clear description of the electrochemical mechanism, identifying the formation of poorly crystalline and/or nanosized KSn at the end of the potassiation of β-Sn. During depotassiation, the formation of the intermediate phase K4Sn9 is established on the basis of DFT and Mössbauer spectroscopy. When tin is associated with antimony in SnSb, a different potassiation path is revealed for tin, with a huge impact on the overall performance. In fact, while the presence of antimony suppresses completely the decomposition of the electrolyte caused by tin particles, the new electrochemical potassiation/depotassiation mechanism drastically reduces the modifications in the local environment and the electrode morphology as evidenced by ex situ and post-mortem SEM analyses. Thanks to the positive impact of the association of tin with antimony, which reduces electrode degradation, a stable high specific capacity of more than 300 mA h g−1 can be achieved for Sn-based negative electrodes in K-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...