Chestnut-like copper cobalt phosphide catalyst for all-pH hydrogen evolution reaction and alkaline water electrolysis†
Abstract
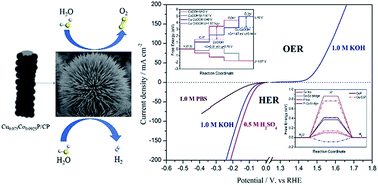
A novel type of chestnut-like copper cobalt phosphide (CuxCo1−xP) nanoarray on carbon fiber paper (CP) for the splitting of water was synthesized through a simple hydrothermal reaction followed by in situ phosphorization treatment. As a hydrogen evolution reaction (HER) catalyst, CuxCo1−xP/CP showed high HER activity at all pH values. To drive a current density of 10 mA cm−2, the optimized Cu0.075Co0.925P/CP required an overpotential of 47, 120, and 70 mV in acidic, neutral, and alkaline media, respectively. In addition, Cu0.075Co0.925P/CP exhibited excellent activity for the oxygen evolution reaction (OER) with a small overpotential of 221 mV to reach 10 mA cm−2. Furthermore, when Cu0.075Co0.925P/CP was used as both the cathode and anode for overall water splitting in 1.0 M KOH, the two-electrode electrolyzer only needed a cell voltage of 1.55 V to achieve 10 mA cm−2, which is superior to that of the noble metal-based Pt/C‖IrO2 cell and most previously reported electrocatalysts. Moreover, this electrolyzer could be powered by a single AA battery with a voltage of 1.5 V. Density functional theory (DFT) calculations further proved that the good catalytic activity of CuxCo1−xP/CP resulted from its smaller hydrogen adsorption free energy (ΔGH*) and overpotential. This work provides a promising strategy to design high-performance and low-cost electrocatalysts for overall water splitting and other energy-related applications.


 Please wait while we load your content...
Please wait while we load your content...