MoCl5 as a dual-function redox mediator for Li–O2 batteries†
Abstract
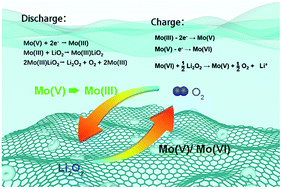
Li–O2 batteries suffer from high polarization and low energy density partly due to the film-like insulating product of Li2O2. These problems can be alleviated by a redox mediator, which usually catalyses either the formation or decomposition of solid Li2O2. However, few redox mediators have dual functions and catalyse both discharge and charge processes. Here, we propose MoCl5 as a dual-function redox mediator for Li–O2 batteries. In the discharge process, with MoCl5, Li2O2 is initially formed in the solution and then deposited on the cathode surface in toroidal morphology, leading to a discharge capacity 2.6 times that without MoCl5. In the charge process, MoCl5 could help oxidize Li2O2 effectively, resulting in an extremely high coulombic efficiency and long cycling lifespan. Therefore, both oxygen reduction and evolution reactions could be promoted greatly for Li–O2 batteries with MoCl5 as the dual-function redox mediator.


 Please wait while we load your content...
Please wait while we load your content...