Pseudo-capacitive behavior induced dual-ion hybrid deionization system based on Ag@rGO‖Na1.1V3O7.9@rGO†
Abstract
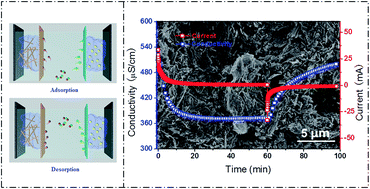
To break the limitation of salt removal capacity of capacitive desalination (CDI), a novel dual-ion hybrid CDI (Di-HCDI) system is proposed which is composed of Na1.1V3O7.9@reduced graphene oxide (NVO@rGO) acting as the sodium ion intercalation electrode while Ag@rGO served as the chloride ion intercalation electrode. Once a direct voltage is applied, the faradaic redox reactions trigger on both electrodes, resulting in sodium ions and chloride ions being removed from the salty electrolyte. NVO exhibits a ribbon structure, having a high sodium intercalation capacity. By coupling with rGO, an ion conductor network is introduced, providing high sodium ion mobility as well as improving the structural stability due to the flexibility of rGO. As a result, the Ag@rGO‖NVO@rGO device demonstrates a superior desalination performance, i.e. ultra-high desalination capacity, rate capability and regeneration. When it was operated in NaCl solution with an initial concentration of 2000 mg L−1 at 1.4 V, the salt removal capacity was 82.2 mg g−1. Remarkably, the charge efficiency of the Ag@rGO‖NVO@rGO device reached 94.4% even in highly concentrated brine, indicating the co-ion effect has been restricted. Benefiting from this, the system proves a Di-HCDI strategy which reveals advances over most of the current CDIs.


 Please wait while we load your content...
Please wait while we load your content...