The 3d–5d orbital repulsion of transition metals in oxyhydroxide catalysts facilitates water oxidation†
Abstract
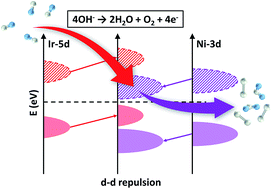
The electrocatalytic oxygen-evolution reaction (OER) is expected to play a vital role in the development of electrochemical energy conversion and storage technologies. 3d transition-metal oxyhydroxides have been reported to outperform noble metal-based catalysts for the OER, but the relatively localized properties of 3d electrons limit the sufficient modulation of their electronic structures by dopants, which may inhibit further improvement of their OER performances. Herein, through density functional theory (DFT) calculation, we found that 5d transition metals such as iridium (Ir) with unique electronic properties can effectively modulate 3d transition-metal oxyhydroxides, thus producing versatile electronic structures to facilitate the OER activity. We therefore synthesized NiFe(3d)Ir(5d) oxyhydroxides and explored their electronic structures via in situ and ex situ X-ray absorption spectroscopy (XAS) and valence band X-ray photoelectron spectroscopy (VB-XPS). The DFT, XAS, VB-XPS and electrochemical studies demonstrated that Ir served as a modulator in the 3d metal oxyhydroxide framework, and created a local environment favoring 3d–5d orbital interaction, and the repelled Ni 3d orbitals facilitated the overall OER process. The Ir-doped catalyst on a glassy carbon electrode delivers 133 mV lower overpotential to achieve a current density of 10 mA cm−2 in an alkaline electrolyte, a 53-fold improved turnover frequency (TOF) over that of pristine NiFe oxyhyroxides, with negligible activity decay after 500 hours of operation.


 Please wait while we load your content...
Please wait while we load your content...