Fundamental insights about interlayer cation migration in Li-ion electrodes at high states of charge†
Abstract
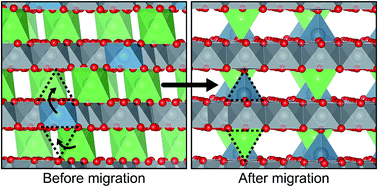
One approach to increasing the capacity of Li-ion batteries is to expand the usable voltage range over which the battery is cycled by charging the cathode to higher voltages. Layered intercalation compounds commonly used as cathodes in Li-ion batteries, however, can become susceptible to irreversible structural changes at high states of charge due to cation migration to the emptied Li layers. Here we report on the discovery of a strong correlation between the position of the Fermi level and the energy barrier for cation migration to the Li layers of fully charged layered intercalation compounds. Since the identity of the transition metal strongly influences the Fermi level, this discovery suggests that cation migration can be suppressed at high states of charge using targeted alloying strategies. First-principles calculations indicate that an increase in the concentration of Ni relative to Co or Mn should reduce, or even impede, interlayer migration in layered oxides. The insights of this study pave the way for the discovery of layered intercalation compounds that can approach their theoretical capacities in practice.


 Please wait while we load your content...
Please wait while we load your content...