Revealing structure–activity links in hydrazine oxidation: doping and nanostructure in carbide–carbon electrocatalysts†
Abstract
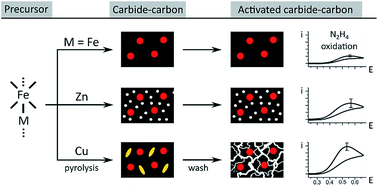
To expand the range of hydrazine oxidation catalysts active in alkaline pH – a key challenge in fuel cell electrocatalysis – we studied the effect of doping on carbide–carbon composites. Pyrolysis of organometallic precursors based on Fe–M cores (M = Fe/Zn/Cu) yielded Fe3C-embedded, N-doped, hierarchically porous, graphitic carbons. These multi-doped materials showed excellent hydrazine oxidation activity (onset 0.28 V vs. RHE at pH = 14). Doping enhanced activity by nanostructural effects, rather than purely catalytic effects, as revealed by electrochemical and material investigations (XRD, XPS, SEM, TEM, Raman and N2 porosimetry). Zinc and copper boosted microporosity in the carbons by two different mechanisms – either gas phase etching by Zn(g) vapors, or enhanced exfoliation of well-dispersed Cu(s) nanoparticles upon reaction with acid.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...