Decoupling half-reactions of electrolytic water splitting by integrating a polyaniline electrode†
Abstract
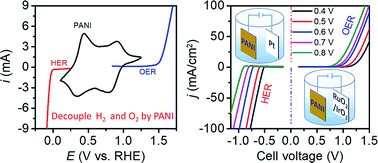
Conventional two-electrode water electrolyzers produce H2 accompanied by O2, and may suffer from gas mixing and high cell voltage inputs. Herein, we introduce a low-cost conducting polymer material polyaniline (PANI) as a solid-state redox mediator for decoupling the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) in acidic media. It allows these two half-reactions to be separated from each other in time during the electrolysis of water. As an intermediary material for charge storage, the PANI electrode exhibits a remarkable reversible discharge capacity of 125 mA h g−1 at a current density of 0.2 A g−1. Moreover, an approach by controlling the equilibrium potential of the PANI electrode is developed to balance the separate cell voltages. In this way, the decoupled HER and OER can be sequentially driven at close cell voltages of only 0.8 V. Considering the low cost of PANI and its excellent electrochemical performance, this study paves the way for the practical application of this state-of-the-art decoupling strategy.


 Please wait while we load your content...
Please wait while we load your content...