Manipulating the water dissociation kinetics of Ni3N nanosheets via in situ interfacial engineering†
Abstract
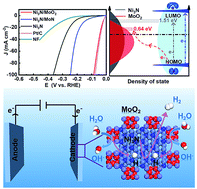
Although Ni3N possesses superior hydrogen desorption behavior, its alkaline hydrogen evolution reaction (HER) catalysis is substantially hindered, due to the high-lying unoccupied orbital center induced sluggish water dissociation kinetics. Herein, we successfully endow Ni3N with exceptional alkaline HER activity by in situ interfacial engineering. The prepared Ni3N/MoO2 interfacial system delivers an ultra-small overpotential of 21 mV at 10 mA cm−2, which is very close to that of the benchmark Pt/C catalysts. Density functional theory (DFT) calculations reveal that MoO2 with a lower unoccupied orbital center could substantially boost the water dissociation kinetics, while the hydrogen desorption proceeds on Ni3N. The capability to understand and design interfacial systems provides an effective pathway for the rational construction of HER catalysts and beyond.


 Please wait while we load your content...
Please wait while we load your content...