Mo-based 2D MOF as a highly efficient electrocatalyst for reduction of N2 to NH3: a density functional theory study†
Abstract
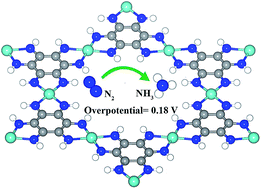
Nitrogen conversion into ammonia is a crucial reaction for many industrial manufacturing processes, but it is a challenging chemical reaction to achieve under ambient conditions. Herein, by using of density functional theory (DFT), we present a conductive metal–organic framework (MOF), which is based on an earth abundant element molybdenum and is used as an electrocatalyst for the nitrogen reduction reaction (NRR). With a highly ordered structure and feasibility in real applications, the Mo-based MOF , among a series newly fabricated 2D MOFs, exhibits an excellent catalytic performance for conversion of N2 into NH3 at room temperature with a very low overpotential of 0.18 V. This report provides atomic level insights to experimental researchers that the newly fabricated 2D MOFs with non-noble metals can be used as an efficient electrocatalyst for the NRR under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...