LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries†
Abstract
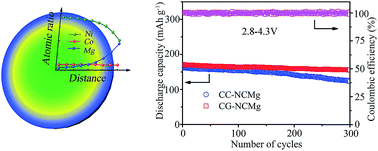
Nickel-rich layered oxides are attractive cathode materials for Li-ion batteries because of high energy density and low cost, but suffer from unsatisfactory cycling performance and poor thermal stability. Here we report the synthesis and application of Mg-concentration-gradient LiNi0.90Co0.07Mg0.03O2 (CG-NCMg) microspheres as a high-performance cathode material. The CG-NCMg, synthesized via Mg-cascade feeding coprecipitation and solid-state lithiation, exhibits a high capacity of 167.4 mA h g−1 at 10C rate and a capacity retention of 80.9% after 300 cycles at 1C, significantly outperforming the Mg-concentration-constant cathode. The superior performance is attributed to the concentration-gradient microstructure, in which the Mg-poor core provides high capacity while the Mg-rich shell with dilated interlayer spacing enhances structure stability and Li-ion diffusivity. These results indicate that low-content, concentration-gradient Mg doping is an efficient strategy to boost Ni-rich layered cathode materials.


 Please wait while we load your content...
Please wait while we load your content...