A multi-functional interface derived from thiol-modified mesoporous carbon in lithium–sulfur batteries†
Abstract
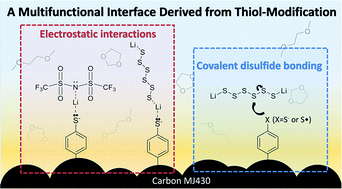
Lithium–sulfur (Li–S) batteries hold great promise as a next-generation energy-storage technology. Their practical application, however, is hindered by the rapid capacity fading associated with the dissolution of lithium polysulfides (LiPSs) into organic electrolytes. In this work, we successfully impede these losses by anchoring thiol (–SH) functional groups to the nonpolar surface of a mesoporous carbon host. This new strategy increases the surface polarity of conductive carbons and traps LiPSs inside cathodes. By utilizing various spectroscopic methods, we investigate the mechanisms of LiPS trapping, which originate from the electrostatic and covalent interactions of the thiol functional groups with Li+ from the electrolyte and with S from the LiPS chains, respectively. Here, we for the first time identify the multiple interactions that are induced by a small molecular interface upon cycling and correlate them with the electrochemical behavior. The fundamental insight on the thiol functionality suggests a further rational design of multi-functional interfaces to achieve better Li–S performance.


 Please wait while we load your content...
Please wait while we load your content...