Atomically tailoring vacancy defects in FeF2.2(OH)0.8 toward ultra-high rate and long-life Li/Na-ion batteries†
Abstract
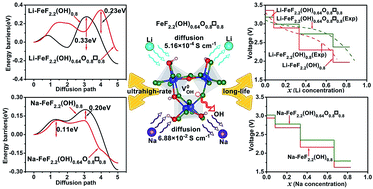
As for FeF2.2(OH)0.8, introducing appropriate vacancy defects has been recently discovered to be a new experimental method for the improvement of the lithium storage performance. However, owing to the limitation of experimental methods, for Li/Na rechargeable batteries, the vacancy formation mechanism of FeF2.2(OH)0.8 and its regulation mechanism on the electrochemical properties associated with rate-performance and cycling stability are still poorly understood. Therefore, we carried out first principles calculations to investigate the defect chemistry in FeF2.2(OH)0.8. Eleven representative vacancy defects, such as neutral iron vacancy (V0Fe), charged iron vacancy (VFe2− and VFe3−), neutral oxygen vacancy (V0O), charged oxygen vacancy (VO+ and VO2+), neutral hydrogen vacancy (V0H), charged hydrogen vacancy (VH−), neutral hydroxide vacancy (V0OH), charged fluorine vacancy (VF+) and neutral fluorine vacancy (V0F) are included. The vacancy formation energies clearly reveal that FeF2.2(OH)0.8 with neutral hydroxide vacancy (V0OH) (FeF2.2(OH)0.64O0.08□0.08) is most likely to form under hydrogen-poor (H-poor) conditions. With the introduction of V0OH, the band gap of FeF2.2(OH)0.8 reduces from 1.47 eV to 0.99 eV, which contributes to the improvement of electronic conductivity. Moreover, by analysis of the defect structure and Li/Na diffusion process, it was proposed that the ion diffusion channel of FeF2.2(OH)0.8 is broadened. Besides, the balance between Li/Na ions and surrounding anions also occurs at the saddle point, which induces higher ionic conductivity to appear in FeF2.2(OH)0.64O0.08□0.08 relative to FeF2.2(OH)0.8 (5.16 × 10−4 S cm−1vs. 1 × 10−6 S cm−1 for Li; 6.88 × 10−2 S cm−1vs. 2.03 × 10−2 S cm−1 for Na). Accordingly, FeF2.2(OH)0.64O0.08□0.08 possesses higher rate performance and more stable discharge voltage than FeF2.2(OH)0.8. As a whole, our theoretical results successfully clarify the origin of the favorable electrochemical properties of FeF2.2(OH)0.64O0.08□0.08 during the Li intercalation/deintercalation process in the experiment. Furthermore, they also clearly simulate the process of Na diffusion kinetics and electrochemistry in FeF2.2(OH)0.8 and FeF2.2(OH)0.64O0.08□0.08. Furthermore, it is verified that introducing V0OH is an effective strategy to design FeF2.2(OH)0.8-based cathode materials for ultra-high rate and long-life Li/Na-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...