Zinc-based spinel cathode materials for magnesium rechargeable batteries: toward the reversible spinel–rocksalt transition†
Abstract
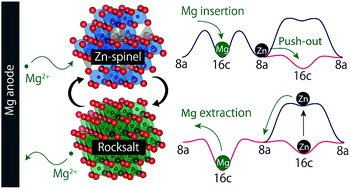
The spinel-to-rocksalt transition with Mg insertion into spinel oxides upon discharge can be utilized as a cathode reaction for magnesium rechargeable batteries. However, the formation of the resulting robust rocksalt phase can be harmful to the cyclability, in that it is sluggish to revert to the original spinel structure. In this work, we show that the inverse “rocksalt-to-spinel” transition can be facilitated upon charge by stabilizing the spinel structure with Zn preferring a tetrahedral environment. Our ab initio calculation study substantiates that Zn-based spinel oxides (space group #227) favor a normal-spinel configuration owing to covalency of Zn–O in the tetrahedral 8a site and cation disordering or migration from 8a to 16c sites tends to be unfavorable in terms of thermodynamics and kinetics. Based on this theoretical prediction, we show experimentally that such a stabilized normal spinel structure (i.e., ZnCo2O4 and ZnFe2O4) consequently allows the reversible spinel–rocksalt transition upon charge and discharge. Furthermore, the volume change of ZnFe2O4 in discharge/charge is much smaller than that of Co-based spinel oxides, which can provide a nearly zero-strain cathode material consisting of abundant elements.


 Please wait while we load your content...
Please wait while we load your content...