The complex defect chemistry of antimony selenide†
Abstract
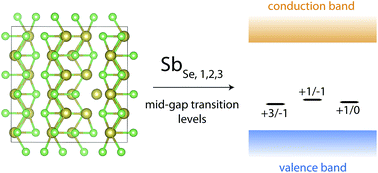
Antimony selenide, Sb2Se3, is a highly promising solar absorber material with excellent optoelectronic properties; solar cell efficiencies are now poised to exceed 10%, after a rapid rise over the past few years. However, the open-circuit voltage (Voc) of most cells remains low, and such a high Voc deficit, along with defect spectroscopy studies, suggest that recombination via deep trap states may be a limiting factor. A comprehensive study of all the intrinsic defects in Sb2Se3 is warranted – in this article, we calculate the formation energies and transition levels of these defects using hybrid Density Functional Theory. Our results demonstrate that cation–anion antisite defects have low formation energies, and possess multiple mid-gap transition levels, making them the most likely candidates for previously observed trap states, and possible recombination centres. Suppressing these dominant defects will be crucial for future cell development – thus we also present potential methods to counteract their detrimental effects and allow further improvement in efficiencies.


 Please wait while we load your content...
Please wait while we load your content...