Novel acetic acid induced Na-rich Prussian blue nanocubes with iron defects as cathodes for sodium ion batteries†
Abstract
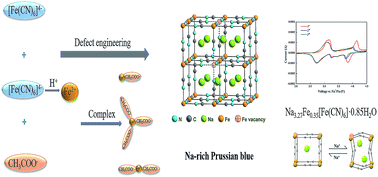
The Prussian blue cathode has great potential for use in sodium-ion batteries in view of its high gravimetric capacity, facile synthetic procedure and low cost. The main challenges for Prussian blue are the structural degradation caused by [Fe(CN)6] vacancies and coordinated water in its lattice and low average voltage due to insufficient activation of low-spin FeLS(C) redox-couple reactions. Here, Na-enriched Prussian blue with low coordinated water and free [Fe(CN)6] vacancies has been successfully synthesized by defect engineering, using acetic acid as an iron defect inducer. In particular, Na-rich Na3.27Fe0.35[Fe(CN)6]·0.85H2O nanocubes with hole centres, low amounts of coordinated water and free [Fe(CN)6] vacancies exhibit a high specific capacity, impressive cycling stability and good coulombic efficiency. This Na-rich material shows a low charge-transfer resistance (201.1 Ω), a high Na+ apparent diffusion coefficient (3.56 × 10−11 cm2 s−1) and an additional capacity contribution at approximately 4.1 V, demonstrating the sufficient activation of low-spin FeLS(C) redox couples in Na-involved reactions. The Na3.27Fe0.35[Fe(CN)6]·0.85H2O cathode undergoes a reversible redox reaction, which converts the structure from cubic Na2Fe0.35[Fe(CN)6] to rhombohedral Na3.24Fe0.35[Fe(CN)6]. More significantly, this work for the first time realizes the rational composition and architecture design of Prussian blue materials by defect engineering for a broad range of potential applications.


 Please wait while we load your content...
Please wait while we load your content...