Effect of trace hydrofluoric acid in a LiPF6 electrolyte on the performance of a Li–organic battery with an N-heterocycle based conjugated microporous polymer as the cathode†
Abstract
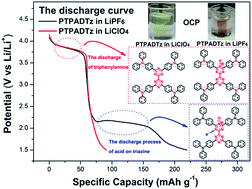
The common LiPF6 electrolyte in lithium batteries often contains trace water (∼10 ppm) and hydrofluoric acid (∼20 ppm). But the possible influence of this trace HF on the performance of Li–organic batteries with organic cathode materials is still not clear. In this paper, a novel N-heterocycle based conjugated microporous polymer PTPADTz based on the triphenylamine–bitriazine group was prepared. The polymer PTPADTz was found to possess a high surface area (∼657 m2 g−1) and an abundant microporous structure. Using PTPADTz as the cathode material in lithium batteries with the LiPF6 electrolyte, apart from the voltage platform of triphenylamine around 3.75 V, a new and unstable low voltage platform at ∼2 V was also observed, which did not correspond to the n-doping process of bitriazine groups according to electrochemistry results. Furthermore, it faded rapidly and disappeared in 20 cycles with an irreversible capacity loss. Such an uncommon phenomenon was not observed in the same lithium batteries with the LiClO4 electrolyte. UV-vis spectra and electrochemistry results showed that the PTPADTz film exhibited an obvious red-shift in the LiPF6 electrolyte similar to that in HCl solution and the HF/LiClO4 electrolyte, with the film color in both changing from yellow to a shade of red, which were not observed in the LiClO4 electrolyte. Thus the uncommon low voltage platform at ∼2 V in the LiPF6 electrolyte might be ascribed to the acid-doping effect of the trace HF in the electrolyte, which bonded with the electron pairs of nitrogen in triazine to increase its electron affinity and produce another new n-doping behavior. The open lithium battery experiments clearly demonstrated the in situ color change related to the doping and dedoping behavior during the whole charge/discharge process, further confirming that the acid-doping of the trace HF into the triazine group was the origin of the observed low voltage platform. Hence, this study may provide new insight into the influence of trace H+ in the electrolyte on the performance of Li–organic batteries.


 Please wait while we load your content...
Please wait while we load your content...