The energetics of phosphoric acid interactions reveals a new acid loss mechanism†
Abstract
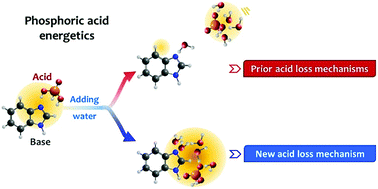
Acid retention of phosphoric acid (PA)-doped proton exchange membranes (PEMs) is one of the critical factors that determine the durability of high temperature PEM fuel cells. However, the mechanism of PA loss in the PEMs in the presence of water is obscure in the context of the energetics of the PA cluster. Here, we study the energetics of PA–benzimidazole acid–base and biphosphate–ammonium ion pairs using density functional theory calculations and 31P NMR experiments to propose a novel PA loss mechanism. The results suggest that the removal of the PA from the membrane does not occur due to the strong interaction of PA–water, but due to the incapability of the base polymers to hold the water and PA beyond a certain level. Significantly higher interaction in the biphosphate–ammonium ion pair shifts the equilibrium PA composition in the PA cluster to higher values, which minimizes the PA loss in the presence of water. Introducing high interaction between base, water, and PA molecules provides a path for better high temperature PEM design with excellent acid retention capabilities.


 Please wait while we load your content...
Please wait while we load your content...