Self-assembled propylammonium cations at grain boundaries and the film surface to improve the efficiency and stability of perovskite solar cells†
Abstract
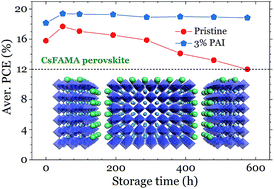
The major challenge of bringing organic–inorganic hybrid perovskite solar cells towards commercialization is their inherent instability especially with regard to moisture. The introduction of a small amount of hydrophobic cations of large size into a 3D perovskite structure which typically aids in the formation of a quasi-2D perovskite structure has been shown to be a promising method for maintaining high efficiency while introducing a high stability toward moisture. By comparing a family of different cations, the propylammonium (PA) cation is found to be the most effective in mitigating these instability issues. Via femtosecond transient absorption and reflection spectroscopy, the large cations are found to preferentially accumulate at the grain boundaries and film surface, forming heterostructures and preventing moisture penetration. The preferential crystal orientation, crystallization, and grain size in both lateral and vertical directions are enhanced accordingly, in particular when PA is incorporated into the perovskite active layer. Such self-assembled large cations suppress charge recombination and enhance device efficiency. Along with enhancements to efficiency, the addition of the PA cation significantly improves both device and precursor stabilities.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...