Maximization of sodium storage capacity of pure carbon material used in sodium-ion batteries
Abstract
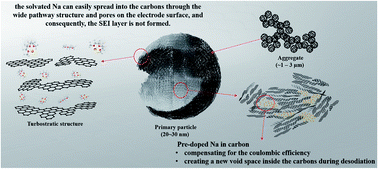
Generally, carbon anode materials used in sodium-ion batteries do not exhibit good electrochemical performance because of low coulombic efficiency (CE). This paper presents a strategy to overcome this limitation by causing a co-intercalation reaction in a newly designed material. Here, Na was doped inside carbons and desodiation was caused by cleaning the doped Na. Consequently, the CE consistently exceeded 100%. Furthermore, new spaces were created when the doped Na was released continuously from the carbons, thereby allowing more Na to be stored in these spaces. This consistently increased the reversible capacity during cycling. Even though the designed material was a nanomaterial with a large specific surface area, the CE in the first cycle was 85%. Because of the co-intercalation reaction, a solid-electrolyte interphase (SEI) layer might not be formed depending on the anode surface structure and continuous long-term stable cycling was possible even without an SEI layer. Thus, a useful material for sodium-ion batteries can be designed using only carbons and without next-generation materials.


 Please wait while we load your content...
Please wait while we load your content...