Oxygen-deficient titanium dioxide as a functional host for lithium–sulfur batteries†
Abstract
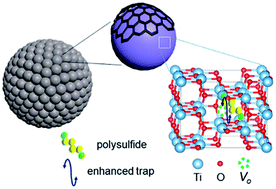
The shuttling of polysulfides with sluggish redox kinetics has severely retarded the advancement of lithium–sulfur (Li–S) batteries. In this work oxygen-deficient titanium dioxide (TiO2) has been investigated as a novel functional host for Li–S batteries. Experimental and first-principles density functional theory (DFT) studies reveal that oxygen vacancies 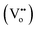 help to reduce polysulfide shuttling and catalyze the redox kinetics of sulfur/polysulfides during cycling. Consequently, the resulting TiO2/S composite cathode manifests superior electrochemical properties in terms of high capacity (1472 mA h g−1 at 0.2C), outstanding rate capability (571 mA h g−1 at 2C), and excellent cycling properties (900 mA h g−1 over 100 cycles at 0.2C). The present strategy offers a viable way through vacancy engineering for the design and optimization of high-performance electrodes for advanced Li–S batteries and other electrochemical devices.
help to reduce polysulfide shuttling and catalyze the redox kinetics of sulfur/polysulfides during cycling. Consequently, the resulting TiO2/S composite cathode manifests superior electrochemical properties in terms of high capacity (1472 mA h g−1 at 0.2C), outstanding rate capability (571 mA h g−1 at 2C), and excellent cycling properties (900 mA h g−1 over 100 cycles at 0.2C). The present strategy offers a viable way through vacancy engineering for the design and optimization of high-performance electrodes for advanced Li–S batteries and other electrochemical devices.


 Please wait while we load your content...
Please wait while we load your content...