The role of substituents in determining the redox potential of organic electrode materials in Li and Na rechargeable batteries: electronic effects vs. substituent-Li/Na ionic interaction†
Abstract
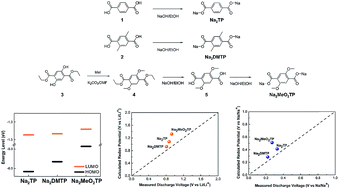
Rechargeable batteries based on organic electrode materials are an attractive energy storage alternative in terms of cost efficiency and sustainability. Feasible chemical modifications of organic materials also offer versatile and easily tunable electrochemical properties to use them as electrodes in battery systems. Herein, we discuss the effect of substituting functional groups on the redox potential of Li- and Na-ion cells using two novel disodium terephthalate (Na2TP) derivatives. It is shown that the substitution of electron donating functional groups generally lowers the discharge voltages of organic anode materials by shifting the lowest unoccupied molecular orbital (LUMO) energy, which is consistent with prior knowledge. In contrast, the same substitution is shown to also increase the voltage owing to specific ion interactions with the substituents. The strong binding interaction between the intercalating ion (Li+) and methoxy substituents significantly lowers the free energy of the discharged products, resulting in elevation of the redox potential despite the high LUMO level of the host molecule. These findings suggest the competition between electronic effects and the ionic interaction as the governing factor determining the redox voltages, providing an important guideline to fine-tune the voltage of new organic electrodes.


 Please wait while we load your content...
Please wait while we load your content...