Self-healing composite polymer electrolyte formed via supramolecular networks for high-performance lithium-ion batteries †
Abstract
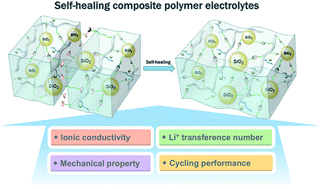
A novel supramolecular network-reinforced and self-healing composite polymer electrolyte (SHCPE) is designed and fabricated via the incorporation of ureido-pyrimidinone (UPy)-functionalized SiO2 (SiO2–UPy) into the polymer matrix containing UPy units. The bonded UPy groups on SiO2 could form supramolecular networks with the polymer via the interactions of quadruple hydrogen bonding. Benefitting from the supramolecular interactions, the fillers of SiO2–UPy are able to uniformly disperse and reduce the agglomeration. As a result, the interphase between SiO2 and the polymer matrix, which is crucial to provide a fast conduction channel for lithium ions, is increased. Consequently, the SHCPE exhibits an enhanced ionic conductivity of 8.0 × 10−5 S cm−1 at 30 °C compared with that of the CPE blended with pristine SiO2. More importantly, the self-healing and mechanical properties of the SHCPE are simultaneously improved due to the increase of physically cross-linked sites in the polymer matrix. Owing to these outstanding properties, the lithium-ion batteries fabricated using the SHCPE exhibit stable cycling performance.


 Please wait while we load your content...
Please wait while we load your content...