Ab initio investigations of orthogonal ScC2 and ScN2 monolayers as promising anode materials for sodium-ion batteries†
Abstract
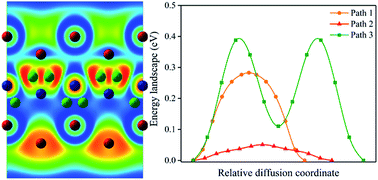
Sodium-ion batteries (SIBs) have attracted widespread intensive attention all over the world owing to their low cost and relatively high safety. The present work reports the first-principles calculations on orthogonal ScC2 and ScN2 monolayers to investigate their feasibility as SIB anode materials. The results show that both o-ScC2 and o-ScN2 monolayers are thermodynamically stable and have metallic features even during the sodiation process. The adsorption energy of one Na ion on 2 × 2 × 1 cells of the monolayer is −0.28 eV for o-ScC2 and −0.75 eV for o-ScN2. Each of o-ScC2 and o-ScN2 has the capacity to accommodate two sodium atoms to form Na2ScC2 and Na2ScN2, which gives low open circuit voltages of 0.08 and 0.10 V, and high Na storage capacities of 777 and 735 mA h g−1, respectively. The ab initio molecular dynamics simulations exhibit the anisotropic diffusion behaviors of Na ions on both o-ScC2 and o-ScN2 monolayers with low energy barriers of 0.050 and 0.269 eV, respectively, which are comparable with those of other outstanding SIB anode materials such as Sc2C (0.012 eV) and MoS2 (0.28 eV). Given the thermodynamic stability, metallic feature, low Na ion diffusion energy barrier, and high specific capacity, both o-ScC2 and o-ScN2 monolayers have great potential to be excellent SIB anode materials.


 Please wait while we load your content...
Please wait while we load your content...