Tailorable surface sulfur chemistry of mesoporous Ni3S2 particles for efficient oxygen evolution†
Abstract
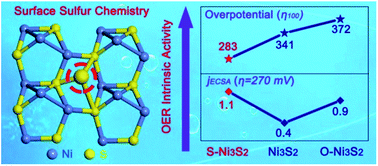
Boosting the intrinsic activity of electrocatalysts is pivotal in enhancing the oxygen evolution reaction (OER) at the source. Herein, we synthesize a mesoporous Ni3S2 particle electrocatalyst on Ni foam that has appropriate surface sulfur chemistry and demonstrates excellent catalytic activity as well as rapid reaction kinetics. The optimized Ni3S2 electrocatalyst shows ultralow overpotentials of 213 and 283 mV at 10 and 100 mA cm−2, respectively, with a very low Tafel slope of 45 mV dec−1 in alkaline media. The ECSA normalized current density is 1.1 mA cm−2 at an overpotential of 270 mV, nearly three times higher than that of pristine Ni3S2 (0.4 mA cm−2). It has been observed that the sulfur-engineered Ni3S2 electrocatalyst can promote more Ni3+ generation with a significant shift of the Ni center binding energy compared with pristine Ni3S2 during the OER. The findings propose a facile tactic to improve the intrinsic OER activity for water splitting by optimizing the surface sulfur chemistry of metal sulfide-based electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...