Reversible Zn-driven reduction displacement reaction in aqueous zinc-ion battery†
Abstract
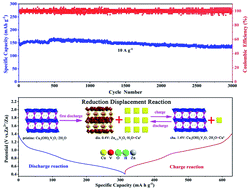
Tremendous attention has been paid to aqueous zinc-ion batteries (ZIBs) with the merits of low cost, safety and environmental benignity. Exploration of the Zn2+ ion storage mechanism is of great significance to the fundamental understanding and future practical application of advanced aqueous Zn-ion battery systems. Herein, we have observed the reduction displacement reaction mechanism upon Zn2+ insertion/extraction into/from the structure of copper pyrovanadate (Cu3(OH)2V2O7·2H2O), i.e., Zn2+ insertion would drive the reduction of Cu2+ to metallic Cu0 particles, and also the phase transition from Cu3(OH)2V2O7·2H2O to a new phase of Zn0.25V2O5·H2O. As a result, Cu3(OH)2V2O7·2H2O is able to deliver excellent electrochemical performance (e.g., a high discharge capacity of 136 mA h g−1 can be maintained after 3000 repetitive cycles at 10 A g−1).


 Please wait while we load your content...
Please wait while we load your content...