Reversible conversion reaction of GeO2 boosts lithium-ion storage via Fe doping†
Abstract
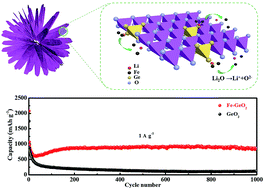
In the present study, for the first time, hierarchically structural microflowers composed of Fe-doped GeO2 nanosheets have been synthesized via a facile solvothermal route. It has been found that Fe3+ was not only a structure-directing agent, facilitating the formation of hierarchical structure by a self-assembly process, but also used for in situ doping of GeO2. As a result, an anode with a hierarchical structure shortened the diffusion distance of Li+ and hierarchically buffer volume expansion. Simultaneously, Fe-GeO2 with oxygen vacancies and narrower bandgaps could provide more active sites for Li insertion and improve the electrical conductivity, respectively. Consequently, Fe-doped GeO2 delivered an ultra-long cycling stability of 866 mA h g−1 even after 1000 cycles at a current density as high as 1 A g−1. It is unexpectedly found that Fe also played a role of catalyst for reversible utilization of partial Li2O.


 Please wait while we load your content...
Please wait while we load your content...