Oxygen vacancy-assisted hydrogen evolution reaction of the Pt/WO3 electrocatalyst†
Abstract
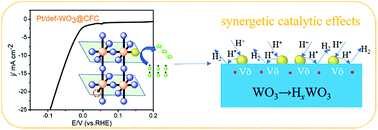
Developing novel hydrogen evolution reaction (HER) catalysts with high activity, high stability and low cost is of great importance for the ever-broader applications of hydrogen energy. Among the conventionally used platinum-based heterogeneous catalysts, the high consumption but low utilization efficiency of precious platinum is one of the most crucial issues. Herein we present a facile approach to prepare an effective HER catalyst with platinum atom clusters highly dispersed in WO3@CFC (carbon fiber cloth). The fabricated Pt/def-WO3@CFC electrocatalyst shows an overpotential of 42 mV at 10 mA cm−2 for the HER in 0.5 M H2SO4, which is highly comparable to that of the state-of-the-art commercial Pt/C catalysts (34 mV), and more attractively, largely enhanced mass activity of Pt and excellent electrochemical stability compared to that of commercial Pt/C. The excellent HER performance of Pt/def-WO3@CFC is attributed to the synergetic catalytic effect between highly dispersed Pt atom clusters and WO3 nanoplates with oxygen vacancies, which enhances both electrocatalytic activity and durability.


 Please wait while we load your content...
Please wait while we load your content...