Amphoteric behavior of hydrogen (H+1 and H−1) in complex hydrides from van der Waals interaction-including ab initio calculations
Abstract
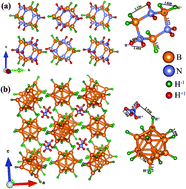
In order to identify potential hydrogen storage materials, worldwide attention has been focused on hydrides with high gravimetric and volumetric capacity. Hydrogen is a unique element that possesses positive, negative or neutral oxidation states in solids depending upon the chemical environment. If one can find hydrogen storage materials where hydrogen is present in both negative and positive oxidation states within the same structural framework then one can accommodate hydrogen with high volume density. So, it is fundamentally as well as technologically important to identify compounds in which hydrogen is amphoteric in nature and understand the necessary criteria for its origin. The experimental structural analysis of cyclotriborazane and diammonium dodeca hydro-closo-dodecaborate insinuates the presence of hydrogen with anionic and cationic behavior within the same structure. In order to understand the role of van der Waals (vdW) interactions on structural parameters, we have considered 12 different vdW corrected functionals and found that the optPBE-vdW functional predicts the equilibrium structural parameters reliably with less than 0.009% error. So, the optPBE-vdW functional is used to calculate charge density, electron localization function, total and partial density of states, Bader and Born effective charge, etc. We observed that hydrogen exhibits amphoteric behavior with H closer to B in a negatively charged state and that closer to N in a positively charged state.


 Please wait while we load your content...
Please wait while we load your content...