A heterogeneous single Cu catalyst of Cu atoms confined in the spinel lattice of MgAl2O4 with good catalytic activity and stability for NO reduction by CO†
Abstract
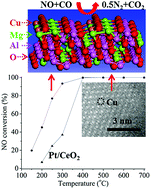
A heterogeneous single Cu catalyst of Cu atoms confined in the spinel lattice of MgAl2O4 (Cu1-MgAl2O4) was prepared by a facile method. Cu1-MgAl2O4 exhibits catalytic activity for NO reduction by CO that is much higher than that of 1.0 wt% Pt/CeO2. Moreover, Cu1-MgAl2O4 exhibits good catalytic stability at high reaction temperatures up to 700 °C during the long-term durability tests. The good stability is attributed to the confinement effect of the stable spinel lattice of Cu1-MgAl2O4 that stabilizes the confined Cu atoms. The high catalytic activity is attributed to the fact that oxygen bonding to Cu atoms of Cu1-MgAl2O4 participates in the oxidation of adsorbed CO with low activation energy, and the adsorption of NO on the resultant oxygen vacancies leads to the formation of N2O with low activation energy, which subsequently decomposes to N2.
- This article is part of the themed collection: International Year of the Periodic Table : Single Atoms as Active Catalysts


 Please wait while we load your content...
Please wait while we load your content...