Applied potential-dependent performance of the nickel cobalt oxysulfide nanotube/nickel molybdenum oxide nanosheet core–shell structure in energy storage and oxygen evolution†
Abstract
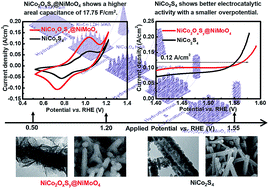
Nickel cobalt sulfide is widely applied to energy storage and electrocatalysis owing to its high electrical conductivity and multiple oxidation states. This study proposes NiCo2S4 and NiCo2OxSy@NiMoO4 core–shell structures as energy storage materials and electrocatalysts for the oxygen evolution reaction. A higher specific capacity of 2.22 mA h cm−2 (168.18 mA h g−1) at 10 mA cm−2 is obtained for the NiCo2OxSy@NiMoO4 electrode which is applied as the battery-type electrode in the battery supercapacitor hybrid, but a better electrocatalytic activity is achieved for the NiCo2S4 electrode with a smaller overpotential of 1.567 VRHE at 0.12 A cm−2 and a smaller Tafel slope of 86.1 mV dec−1. The electrochemically active surface area (ECSA) is larger for NiCo2S4 due to the nanoparticle-assembled nanotube wall, whereas NiCo2OxSy@NiMoO4 shows higher electrical conductivity owing to the presence of molybdenum. Different performances in energy storage and electrocatalysis for NiCo2S4 and NiCo2OxSy@NiMoO4 nanomaterials are caused by different potentials applied for driving the electrochemical reactions. This study for the first time proves that the trade-off between ECSA and electrical conductivity is important for designing nanomaterials applied in different electrochemical fields.


 Please wait while we load your content...
Please wait while we load your content...