Boosting electrochemical water splitting via ternary NiMoCo hybrid nanowire arrays†
Abstract
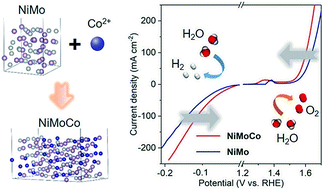
Hydrogen production by electrochemical water splitting is a technology with the potential to meet the growing worldwide demand for sustainable and clean energy. However, the development of cost-effective catalysts to replace noble metals, such as platinum or ruthenium, remains crucial for large-scale hydrogen production. This study presents the synthesis of a transition non-noble metal-based ternary NiMoCo hybrid nanowire array as an efficient bifunctional electrocatalyst for overall water splitting in 1.0 M KOH electrolyte. The catalyst exhibits a low cell voltage of 1.56 V to achieve a water-splitting current density of 10 mA cm−2 together with long-term stability with only 5% of the initial current lost after 100 hours. X-ray absorption spectroscopy confirms that the addition of Co to the binary Ni–Mo system results in a highly mixed chemical binding state with modulated electronic structures. Density functional theory (DFT) calculations reveal that the Co atoms on the ternary alloy become catalytically active sites and facilitate adsorption of intermediates by ensuring preferable interactions between the reactants and the catalyst surface in comparison to the binary counterpart. This work provides a new direction along which to activate binary alloys to further enhance their catalytic abilities in overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...