NaCl-induced nickel–cobalt inverse spinel structure for boosting hydrogen evolution from ethyl acetate and water†
Abstract
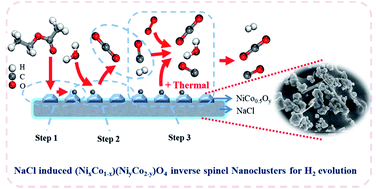
The proper design of an NaCl-induced nickel–cobalt inverse spinel structure is reported as a promising catalyst for boosting H2 evolution from the energy benign sources of ethyl acetate and water. The designed NiCo0.5Oy/NaCl catalyst exhibits the optimal performance with ∼100% EA conversion, 88.1% H2 selectivity and high stability during autothermal reforming at 650 °C and achieves a very high H2 selectivity of 96.3% at 600 °C by accelerating the water–gas shifting reaction (the rate-determining step). The multiple (NixCo1−x)(NiyCo2−y)O4 inverse spinel structures play significant roles in the enhanced catalytic performance. Benefiting from the unique advantages of (i) stable inverse spinel structures, (ii) abundant domains and defects, (iii) abnormal Ni2+/Ni3+ (0.36) and Co2+/Co3+ (3.03) ratios, and (iv) rich redox ability, the catalyst possesses high adsorption capacity towards EA and H2O, abundant active sites and fast electron exchange ability between the reactants and the catalyst. Consequently, the catalyst exhibits a highly efficient and robust hydrocarbon fuel reforming performance. These findings will lead to the development of novel catalysts based on inverse spinels for hydrogen production applications.


 Please wait while we load your content...
Please wait while we load your content...