Effects of LiBOB on salt solubility and BiF3 electrode electrochemical properties in fluoride shuttle batteries†
Abstract
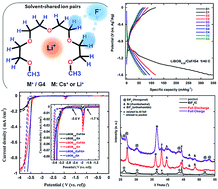
In this study, lithium bis(oxalato)borate (LiBOB) was used for the first time in a fluoride shuttle battery (FSB) to overcome the solubility problem of fluorine-based salts typically present in organic solvents. For this purpose, tetraglyme (G4) electrolytes containing CsF salt and LiBOB with three different concentrations (LiBOB0.06/CsF/G4, LiBOB0.25/CsF/G4, and LiBOB0.5/CsF/G4) were prepared. The effects of LiBOB on the electrochemical compatibility of the bismuth fluoride positive electrode were examined by cyclic voltammetry, charge–discharge tests, and alternating current impedance measurements. The related discharge and charge reactions were confirmed by X-ray diffractometry, whereas 19F NMR and Raman spectroscopies were used to detect potential interactions in the various LiBOB/CsF/G4 systems. At the lowest and highest LiBOB concentrations (i.e., in LiBOB0.06/CsF/G4 and LiBOB0.5/CsF/G4, respectively), the electrolyte decomposition was dominant, whereas the intermediate concentration in LiBOB0.25/CsF/G4 was found to be the optimum condition and played a critical role in CsF solubility, allowing a successful fluoride shuttle-based redox reaction.


 Please wait while we load your content...
Please wait while we load your content...