Kinetic phases of Ag–Cu alloy films are accessible through photodeposition†
Abstract
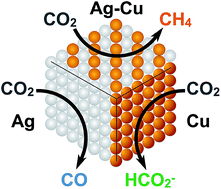
Silver and copper are each widely-studied metals for catalyzing the CO2 reduction reaction (CO2RR), yet studies of Ag–Cu alloys are very rare due to the immiscibility of the two metals. Reported herein is a synthetic route that provides access to Ag–Cu alloys at ambient pressures and temperatures. This method involves solution-depositing a homogeneous mixture of silver(I) 2-ethylhexanoate and copper(II) bis-(2-ethylhexanoate) on a substrate, and photolyzing the thin films with ultraviolet light in an inert dinitrogen atmosphere. This photodeposition procedure is shown to furnish metastable phases of alloys with ∼10 atomic weight% (at%) copper incorporated into the silver lattice. The CO2RR activity of the alloyed sample shows a preference for forming CH4 rather than CO or HCO2− favored for our pure silver and copper films, respectively. These results provide proof that photodeposition can be used to access kinetic phases of alloys that are useful for heterogeneous reaction chemistry.


 Please wait while we load your content...
Please wait while we load your content...