Bifunctional OER/ORR catalytic activity in the tetrahedral YBaCo4O7.3 oxide†
Abstract
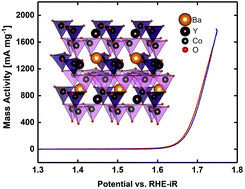
Transition metal oxides, mostly perovskites, have received attention as effective catalysts for the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) in alkaline media. However, despite the numerous reaction mechanisms and descriptors proposed, the understanding of the nature of their electrochemical activity is still far from complete. Herein, we present YBaCo4O7.3 oxide with tetrahedrally coordinated Co2+/Co3+ as a fundamentally new bifunctional catalyst, which demonstrates OER activity of 1.18 mA cm−2 at an overpotential of 400 mV and ORR activity of 2.76 mA cm−2 at an overpotential of −537 mV, exceeding that of the LaNiO3 benchmark catalyst. The electrochemical activity of YBaCo4O7.3 was rationalized in terms of the Zaanen–Sawatzky–Allen model using DFT-based calculations. According to the results, overstoichiometric oxygen induces octahedral Co positions as active sites in the OER, while large Coulomb on-site repulsion energy for Co in the tetrahedral coordination makes these sites active in the ORR.


 Please wait while we load your content...
Please wait while we load your content...