Controllable charge capacity using a black additive for high-energy-density sodium-ion batteries†
Abstract
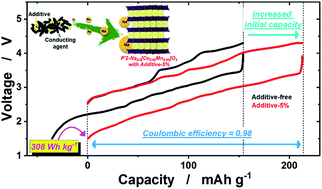
Sodium-deficient P2 or P′2 type layered materials are known to deliver high capacity with acceptable capacity retention. However, the initial charge capacity is substantially lower than the discharge capacity because of the insufficient amount of sodium in their crystal structure, hindering practical application of these materials as cathodes in sodium-ion batteries (SIBs). This limitation can be overcome by introducing a sacrificial salt additive, which participates in the electrochemical oxidation reaction by releasing enough sodium ions to compensate for the insufficient sodium content in the cathode material. Herein, the sacrificial salt NaNO2 was blended with a high-capacity orthorhombic P′2 type Na2/3[Co0.05Mn0.95]O2 cathode material, increasing the initial charge capacity from 154 to 210 mA h g−1. During electrochemical oxidation, the NaNO2 was oxidatively decomposed by the following reaction: NaNO2 → NO2 + Na+ + e−, where NO2 is an oxidizer that enables full desodiation to Na0[Co0.05Mn0.95]O2. The first coulombic efficiency of Na2/3[Co0.05Mn0.95]O2 was improved from 1.38 to 0.98 by virtue of the sacrificing and oxidizing roles of NaNO2. These findings demonstrate that the introduction of NaNO2 as an additional sodium source in cathodes can open new opportunities for the adoption of sodium-deficient cathode materials in practical SIBs.


 Please wait while we load your content...
Please wait while we load your content...