Dolomite: a low cost thermochemical energy storage material†
Abstract
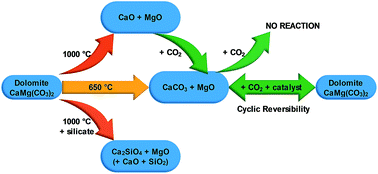
The thermal energy storage properties of dolomite, CaMg(CO3)2, from three sources (commercial, mined, and synthetic) are investigated as a potential solid–gas thermochemical energy store for concentrating solar thermal power (CSP) plants. The reversible carbon dioxide release/absorption cycle can be used to store and release large quantities of thermal energy that can be harnessed near 550 °C. To enable carbon dioxide absorption, a novel molten salt eutectic NaCl : MgCl2 mixture was added to the dolomite, which proved effective. Curiously, the mined (unrefined) sample proved to have the best calcination/carbonation properties with a stable cyclic stability of ∼50 mol% CO2 over 10 cycles. A morphological investigation by scanning electron microscopy reveals the mined sample contains impurities (e.g. quartz) that prevent detrimental agglomeration of dolomite and its reaction products (CaCO3 and MgO). High levels of porosity in the mined dolomite also provide short gas–solid reaction pathways during carbonation. A commercial dolomite source contained too many impurities, resulting in the formation of high levels of Ca2SiO4 that acted as a Ca-sink, lowering CO2 capacity. Finally, a high purity synthetic dolomite sample displayed high levels of agglomeration and phase segregation, lowering reversible CO2 capacity, which was attributed to a lack of impurities that restrict agglomeration.


 Please wait while we load your content...
Please wait while we load your content...