Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes†
Abstract
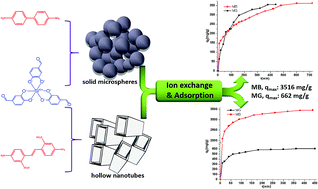
We report straightforward access to two new anionic porous organic polymers (Si-POPs) based on hexacoordinate [SiO6]2– species and polyimine aromatic linkers which exhibit outstanding capabilities for removal of cationic dyes from contaminated industrial water. These multifunctional materials are stable under air and in water; they were synthesized via solvothermal chemistry exploiting Schiff base formation between tri(protocatechuic aldehyde)-silicate and 4,4′-diaminobiphenyl acid (for Si-POP-1) or 4,4′-diamino-2,2′-stilbenedisulfonic acid (for Si-POP-2). Charge neutrality of the Si-POPs was achieved by [Et3NH]+ cations. The structures were determined by spectroscopic techniques (FT-IR and solid 13C-NMR spectroscopy), while SEM and TEM revealed a micrometer-scale solid sphere morphology for Si-POP-1 and hollow nanometer-sized tubes for Si-POP-2. BET specific surface area determination gave 376 and 234 m2 g−1 for Si-POP-1 and Si-POP-2, respectively. The unprecedented adsorption abilities of these materials for standard cationic dyes such as methylene blue (MB), malachite green (MG), and Basic Blue 7 (BB7) were revealed by studying the effects of system variables such as pH, contact time, temperature and mixed-dye solutions. At 25 °C, the maximum adsorption capacity of Si-POP-1 reached 300.3 mg g−1 for MB and 378.5 mg g−1 for MG, while the performance of Si-POP-2 at the same temperature was substantially higher (3516 mg g−1 for MB and 662 mg g−1 for MG). Furthermore, at 35 °C, the maximum adsorption of MB on Si-POP-2 reached 4098 mg g−1, surpassing the performance of previous adsorbents. It was found that both Si-POP-1 and Si-POP-2 exhibit charge and size-selective adsorption. Thermodynamics studies indicated that the adsorptions were spontaneous and endothermic. A minimal loss of adsorptive capacity of Si-POP-2 (less than 4%) was observed after four cycles, demonstrating an effective reuse of the regenerated POP in discarding MB from effluents. Importantly, this green protocol also allows recovery of the dye retained in the POP. These assets recommend these new hexacoordinate silicate-based adsorbents as viable materials for wastewater remediation by removal of organic dye pollutants affecting nature and public health.


 Please wait while we load your content...
Please wait while we load your content...