Preparation and characterization of β-casein stabilized lipopeptide lyotropic liquid crystal nanoparticles for delivery of doxorubicin†
Abstract
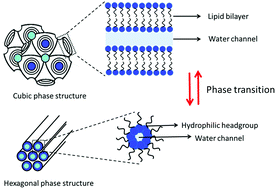
A kind of lyotropic liquid crystal nanoparticle (LLC NPs) has been designed and prepared. LLC NPs are dSMO/OA/β-casein/water quaternary systems, and their cubic or hexagonal microstructures have been characterized by cryogenic transmission electron microscopy (cryo-TEM) and small angle X-ray scattering (SAXS). The phase transition of LLC NPs takes place with ratio and pH adjustments. The properties, such as cytotoxicity, stability, drug encapsulation and release ability, have been investigated with MTT assay, cryo-TEM and UV-Vis spectroscopy. The results showed that LLC NPs were nontoxic to cells and stable to enzymatic degradation. Hydrophilic drug doxorubicin hydrochloride (DOX·HCl) could be effectively encapsulated in LLC NPs and its release rate could be regulated by pH. It was concluded that LLC NPs are potential nanocarriers in nanomedicine technologies. We hope that this work provides new guidelines for the rational design of LLC NP systems with lipopeptides for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...