Diffusion of multiple electrolytes cannot be treated independently: model predictions with experimental validation†
Abstract
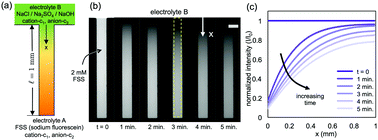
We study the diffusion of multiple electrolytes in a one-dimensional pore. We model the scenario where an electrolyte is in contact with a reservoir of another electrolyte, such that the cation of the two electrolytes is common. The model reveals that several factors influence the ion concentration profiles: (i) relative diffusivities of the ions, (ii) ratio of the electrolyte concentrations in the pore and the reservoir, and (iii) the valence of the ions. We demonstrate that it is crucial to consider the interaction between ion fluxes as treating the electrolytes independently, as is sometimes proposed, does not completely capture the dynamics of ion transport. We validate our numerical predictions by conducting experiments with sodium fluorescein salt in the pore and sodium chloride/sodium sulphate/sodium hydroxide in the reservoir. Our visualization and results demonstrate that ion diffusivities and concentrations in the reservoir can influence the diffusion rates of fluorescein, which underscores that ion fluxes are coupled and that multiple electrolytes cannot be treated independently. These results should be useful to the wide range of situations where concentration variations are imposed on systems with an existing background electrolyte.
- This article is part of the themed collection: Soft Matter Lectureship Winners


 Please wait while we load your content...
Please wait while we load your content...