Chain dynamics and glass transition of dry native cellulose solutions in ionic liquids
Abstract
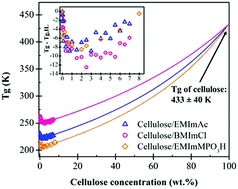
Dry native cellulose solutions in 1-butyl-3-methylimidazolium methylphosphonate (EMImMPO3H), 1-butyl-3-methylimidazolium acetate (EMImAc), and 1-butyl-3-methylimidazolium chloride (BMImCl) ionic liquids (IL) were investigated using subambient linear viscoelastic oscillatory shear. Glass transition temperatures (Tg) of solutions with various cellulose concentrations up to 8.0 wt% were observed as the peaks of loss tangent tan(δ) and loss modulus G′′ in descending temperature sweeps at 1 rad s−1. Cellulose/IL solutions showed a minimum in Tg at ∼2.0 wt% cellulose content before increasing with cellulose concentration, suggesting a perturbation of the strongly structured IL solvents by the cellulose chains. Isothermal frequency sweeps in the vicinity of Tg were used to construct time–temperature-superposition master curves. The angular frequency shift factor aT as a function of temperature indicates Arrhenius behavior within a 9 K range near Tg, allowing calculation of fragility, which was found to be constant up to 8.0 wt% cellulose concentration. This result implied that increasing cellulose concentration initially decreases Tg due to disrupted ionic regularity of ILs, but does not seem to change their fragility.


 Please wait while we load your content...
Please wait while we load your content...