Self-assembly and adsorption of cetyltrimethylammonium bromide and didodecyldimethylammonium bromide surfactants at the mica–water interface
Abstract
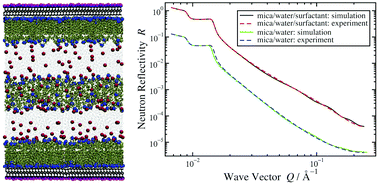
The self-assembly and adsorption of the surfactants cetyltrimethylammonium bromide (CTAB) and didodecyldimethylammonium bromide (DDAB) at the muscovite mica–water interface are studied using molecular-dynamics simulations. Adsorption takes place by an ion-exchange mechanism, in which K+ ions are replaced by the organic alkylammonium cations from the solution. Simulations are performed with and without the surface K+ ions, with pure water, and with the surfactants in aqueous solution. CTAB and DDAB form micellar structures in bulk solution, and in the absence of the surface K+ ions, they quickly adsorb and form bilayer structures. The bilayer ordering of CTAB is not perfect, and there is a competition with the formation of cylindrical micelles. DDAB, on the other hand, forms a well-ordered bilayer structure, with the innermost layer showing strong orientational ordering, and the outermost layer being more disordered. The simulations with pure water highlight the molecular ordering and strong electrostatic interactions with the mica-surface atoms. Using simulated scattering length density profiles, the results are compared directly and critically with existing neutron reflectivity measurements. The simulation results are generally consistent with experiments, and yield new insights on the molecular-scale ordering at the mica–water interface.


 Please wait while we load your content...
Please wait while we load your content...