Competing forces in the self-assembly of amide-functionalized discotic mesogens†
Abstract
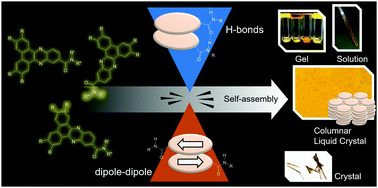
The effect of incorporating a single amide group on the self-assembly of discotic mesogens was examined. Two series of tetraalkoxydibenzophenazines amides were prepared: tertiary diethyl amides, dEt(n) incapable of hydrogen bonding, and secondary amides HBu(n) that can act as both H-bond donors and acceptors. These compounds exhibit markedly different behavior in solution; NMR studies of dEt(n) show no evidence of self-association, whereas HBu(n) strongly associate via H-bonding and π-stacking. Compounds HBu(n) also act as small molecule gelators in a range of solvents, a property not observed for the corresponding tertiary amides. All compounds were found to form Colh liquid crystal phases; variable temperature XRD experiments indicate that each column has a diameter approximately equal to that of a single molecule. A comparison of the phase behavior of HBu(n) and dEt(n) suggests that the columnar phases of the former are stabilized by hydrogen bonding, likely at the expense of local parallel alignment of these molecules. Single crystal X-ray diffraction studies revealed that dEt(6) adopts an antiparallel arrangement in the solid state, in keeping with previous theories of packing within columnar LC phases. These studies highlight the interplay between competing factors, such as hydrogen bonding, π-stacking and dipole–dipole interactions that affect the stability of the LC phases.


 Please wait while we load your content...
Please wait while we load your content...