Self-thermoelectrophoresis at low salinity†
Abstract
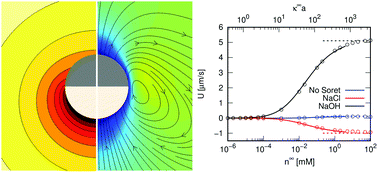
A locally heated Janus colloid can achieve motion in an electrolyte by an effect known as self-thermo(di)electrophoresis. We numerically study the self-propulsion of such a “hot swimmer” in a monovalent electrolyte using the finite-element method and analytic theory. The effect of electrostatic screening for intermediate and large Debye lengths is charted and we report on the fluid flow generated by self-thermoelectrophoresis. We obtain excellent agreement between our analytic theory and numerical calculations in the limit of high salinity, validating our approach. At low salt concentrations, we employ Teubner's integral formalism to arrive at expressions for the speed, which agree semi-quantitatively with our numerical results for conducting swimmers. This lends credibility to the remarkably high swim speed at very low ionic strength, which we numerically obtain for a fully insulating swimmer. We also report on hot swimmers with a mixed electrostatic boundary conditions. Our results should benefit the realization and analysis of further experiments on thermo(di)electrophoretic swimmers.


 Please wait while we load your content...
Please wait while we load your content...